Difference between revisions of "CssR"
| Line 64: | Line 64: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU33010&redirect=T BSU33010] | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/cssRS.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/cssRS.html] | ||
| Line 100: | Line 101: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU33010&redirect=T BSU33010] | ||
* '''Structure:''' | * '''Structure:''' | ||
Revision as of 14:42, 2 April 2014
- Description: two-component response regulator, control of cellular responses to protein secretion stress
| Gene name | cssR |
| Synonyms | yvqA |
| Essential | no |
| Product | two-component response regulator |
| Function | control of cellular responses to protein secretion stress |
| Gene expression levels in SubtiExpress: cssR | |
| Interactions involving this protein in SubtInteract: CssR | |
| Metabolic function and regulation of this protein in SubtiPathways: cssR | |
| MW, pI | 26 kDa, 5.071 |
| Gene length, protein length | 675 bp, 225 aa |
| Immediate neighbours | htrB, cssS |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 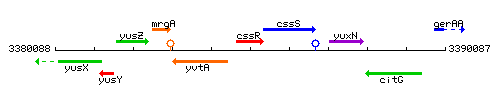
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
transcription factors and their control, heat shock proteins, phosphoproteins
This gene is a member of the following regulons
The CssR regulon:
The gene
Basic information
- Locus tag: BSU33010
Phenotypes of a mutant
Database entries
- BsubCyc: BSU33010
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: OmpR family of two-component response regulators
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylated by CssS on an Asp residue
- Cofactor(s):
- Effectors of protein activity: phosphorylation likely affects DNA-binding activity
- Interactions:
- CssR interacts with the RNA polymerase PubMed
- CssS-CssR
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU33010
- Structure:
- UniProt: O32192
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Olivier Delumeau, François Lecointe, Jan Muntel, Alain Guillot, Eric Guédon, Véronique Monnet, Michael Hecker, Dörte Becher, Patrice Polard, Philippe Noirot
The dynamic protein partnership of RNA polymerase in Bacillus subtilis.
Proteomics: 2011, 11(15);2992-3001
[PubMed:21710567]
[WorldCat.org]
[DOI]
(I p)
Hein Trip, Patricia J van der Veek, Ton C Renniers, Rob Meima, Cees M Sagt, Lisette Mohrmann, Oscar P Kuipers
A novel screening system for secretion of heterologous proteins in Bacillus subtilis.
Microb Biotechnol: 2011, 4(5);673-82
[PubMed:21624103]
[WorldCat.org]
[DOI]
(I p)
Des Raj Kashyap, Minhui Wang, Li-Hui Liu, Geert-Jan Boons, Dipika Gupta, Roman Dziarski
Peptidoglycan recognition proteins kill bacteria by activating protein-sensing two-component systems.
Nat Med: 2011, 17(6);676-83
[PubMed:21602801]
[WorldCat.org]
[DOI]
(I p)
Jessica C Zweers, Thomas Wiegert, Jan Maarten van Dijl
Stress-responsive systems set specific limits to the overproduction of membrane proteins in Bacillus subtilis.
Appl Environ Microbiol: 2009, 75(23);7356-64
[PubMed:19820159]
[WorldCat.org]
[DOI]
(I p)
Hanne-Leena Hyyryläinen, Milla Pietiäinen, Tuula Lundén, Anna Ekman, Marika Gardemeister, Sanna Murtomäki-Repo, Haike Antelmann, Michael Hecker, Leena Valmu, Matti Sarvas, Vesa P Kontinen
The density of negative charge in the cell wall influences two-component signal transduction in Bacillus subtilis.
Microbiology (Reading): 2007, 153(Pt 7);2126-2136
[PubMed:17600057]
[WorldCat.org]
[DOI]
(P p)
Elise Darmon, Ronald Dorenbos, Jochen Meens, Roland Freudl, Haike Antelmann, Michael Hecker, Oscar P Kuipers, Sierd Bron, Wim J Quax, Jean-Yves F Dubois, Jan Maarten van Dijl
A disulfide bond-containing alkaline phosphatase triggers a BdbC-dependent secretion stress response in Bacillus subtilis.
Appl Environ Microbiol: 2006, 72(11);6876-85
[PubMed:17088376]
[WorldCat.org]
[DOI]
(P p)
Elise Darmon, David Noone, Anne Masson, Sierd Bron, Oscar P Kuipers, Kevin M Devine, Jan Maarten van Dijl
A novel class of heat and secretion stress-responsive genes is controlled by the autoregulated CssRS two-component system of Bacillus subtilis.
J Bacteriol: 2002, 184(20);5661-71
[PubMed:12270824]
[WorldCat.org]
[DOI]
(P p)
H L Hyyryläinen, A Bolhuis, E Darmon, L Muukkonen, P Koski, M Vitikainen, M Sarvas, Z Prágai, S Bron, J M van Dijl, V P Kontinen
A novel two-component regulatory system in Bacillus subtilis for the survival of severe secretion stress.
Mol Microbiol: 2001, 41(5);1159-72
[PubMed:11555295]
[WorldCat.org]
[DOI]
(P p)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)