Difference between revisions of "CotA"
(→References) |
|||
| Line 99: | Line 99: | ||
* '''Operon:''' ''cotA'' (according to [http://dbtbs.hgc.jp/COG/prom/cotA.html DBTBS]) | * '''Operon:''' ''cotA'' (according to [http://dbtbs.hgc.jp/COG/prom/cotA.html DBTBS]) | ||
| − | * '''[ | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=cotA_683462_685003_-1 cotA] {{PubMed|22383849}} |
| + | |||
| + | * '''Sigma factor:''' [[SigK]] {{PubMed|15699190,3135411}} | ||
* '''Regulation:''' | * '''Regulation:''' | ||
Revision as of 15:27, 12 April 2012
- Description: laccase, bilirubin oxidase, spore coat protein (outer)
| Gene name | cotA |
| Synonyms | pig |
| Essential | no |
| Product | laccase, bilirubin oxidase |
| Function | resistance of the spore |
| MW, pI | 58 kDa, 5.89 |
| Gene length, protein length | 1539 bp, 513 aa |
| Immediate neighbours | yeaA, gabP |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 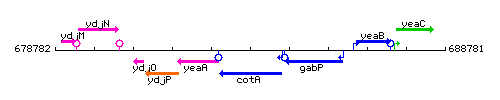
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU06300
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- outer spore coat, more abundant at the mother cell-distal pole of the forespore PubMed
Database entries
- Structure: 2BHF (reduced form)
- UniProt: P07788
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: cotA (according to DBTBS)
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Catarina S Silva, João M Damas, Zhenjia Chen, Vânia Brissos, Lígia O Martins, Cláudio M Soares, Peter F Lindley, Isabel Bento
The role of Asp116 in the reductive cleavage of dioxygen to water in CotA laccase: assistance during the proton-transfer mechanism.
Acta Crystallogr D Biol Crystallogr: 2012, 68(Pt 2);186-93
[PubMed:22281748]
[WorldCat.org]
[DOI]
(I p)
André T Fernandes, Manuela M Pereira, Catarina S Silva, Peter F Lindley, Isabel Bento, Eduardo Pinho Melo, Lígia O Martins
The removal of a disulfide bridge in CotA-laccase changes the slower motion dynamics involved in copper binding but has no effect on the thermodynamic stability.
J Biol Inorg Chem: 2011, 16(4);641-51
[PubMed:21369750]
[WorldCat.org]
[DOI]
(I p)
Isabel Bento, Catarina S Silva, Zhenjia Chen, Lígia O Martins, Peter F Lindley, Cláudio M Soares
Mechanisms underlying dioxygen reduction in laccases. Structural and modelling studies focusing on proton transfer.
BMC Struct Biol: 2010, 10;28
[PubMed:20822511]
[WorldCat.org]
[DOI]
(I e)
Nirupama Gupta, Edgardo T Farinas
Directed evolution of CotA laccase for increased substrate specificity using Bacillus subtilis spores.
Protein Eng Des Sel: 2010, 23(8);679-82
[PubMed:20551082]
[WorldCat.org]
[DOI]
(I p)
Zhenjia Chen, Paulo Durão, Catarina S Silva, Manuela M Pereira, Smilja Todorovic, Peter Hildebrandt, Isabel Bento, Peter F Lindley, Lígia O Martins
The role of Glu498 in the dioxygen reactivity of CotA-laccase from Bacillus subtilis.
Dalton Trans: 2010, 39(11);2875-82
[PubMed:20200715]
[WorldCat.org]
[DOI]
(I p)
Daisuke Imamura, Ritsuko Kuwana, Hiromu Takamatsu, Kazuhito Watabe
Localization of proteins to different layers and regions of Bacillus subtilis spore coats.
J Bacteriol: 2010, 192(2);518-24
[PubMed:19933362]
[WorldCat.org]
[DOI]
(I p)
Leif Steil, Mónica Serrano, Adriano O Henriques, Uwe Völker
Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis.
Microbiology (Reading): 2005, 151(Pt 2);399-420
[PubMed:15699190]
[WorldCat.org]
[DOI]
(P p)
M F Hullo, I Moszer, A Danchin, I Martin-Verstraete
CotA of Bacillus subtilis is a copper-dependent laccase.
J Bacteriol: 2001, 183(18);5426-30
[PubMed:11514528]
[WorldCat.org]
[DOI]
(P p)
L Zheng, R Halberg, S Roels, H Ichikawa, L Kroos, R Losick
Sporulation regulatory protein GerE from Bacillus subtilis binds to and can activate or repress transcription from promoters for mother-cell-specific genes.
J Mol Biol: 1992, 226(4);1037-50
[PubMed:1518043]
[WorldCat.org]
[DOI]
(P p)
K Sandman, L Kroos, S Cutting, P Youngman, R Losick
Identification of the promoter for a spore coat protein gene in Bacillus subtilis and studies on the regulation of its induction at a late stage of sporulation.
J Mol Biol: 1988, 200(3);461-73
[PubMed:3135411]
[WorldCat.org]
[DOI]
(P p)
W Donovan, L B Zheng, K Sandman, R Losick
Genes encoding spore coat polypeptides from Bacillus subtilis.
J Mol Biol: 1987, 196(1);1-10
[PubMed:2821284]
[WorldCat.org]
[DOI]
(P p)