Difference between revisions of "SipS"
| Line 14: | Line 14: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || protein secretion | |style="background:#ABCDEF;" align="center"|'''Function''' || protein secretion | ||
(modulation of the capacity and specificity for protein secretion) | (modulation of the capacity and specificity for protein secretion) | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/wiki/index.php/Protein_secretion Protein secretion]''' | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 20 kDa, 9.718 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 20 kDa, 9.718 | ||
Revision as of 13:45, 11 December 2010
- Description: signal peptidase I
| Gene name | sipS |
| Synonyms | |
| Essential | no |
| Product | signal peptidase I |
| Function | protein secretion
(modulation of the capacity and specificity for protein secretion) |
| Metabolic function and regulation of this protein in SubtiPathways: Protein secretion | |
| MW, pI | 20 kDa, 9.718 |
| Gene length, protein length | 552 bp, 184 aa |
| Immediate neighbours | ypuD, ypzC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 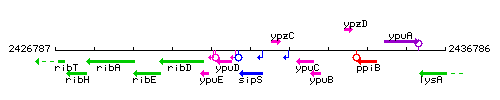
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU23310
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Cleavage of hydrophobic, N-terminal signal or leader sequences from secreted and periplasmic proteins (according to Swiss-Prot)
- Protein family: peptidase S26 family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- UniProt: P28628
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: sipS PubMed
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Jan Maarten van Dijl, Groningen, Netherlands
Your additional remarks
References
A Bolhuis, H Tjalsma, K Stephenson, C R Harwood, G Venema, S Bron, J M van Dijl
Different mechanisms for thermal inactivation of Bacillus subtilis signal peptidase mutants.
J Biol Chem: 1999, 274(22);15865-8
[PubMed:10336490]
[WorldCat.org]
[DOI]
(P p)
H Tjalsma, A Bolhuis, M L van Roosmalen, T Wiegert, W Schumann, C P Broekhuizen, W J Quax, G Venema, S Bron, J M van Dijl
Functional analysis of the secretory precursor processing machinery of Bacillus subtilis: identification of a eubacterial homolog of archaeal and eukaryotic signal peptidases.
Genes Dev: 1998, 12(15);2318-31
[PubMed:9694797]
[WorldCat.org]
[DOI]
(P p)
H Tjalsma, M A Noback, S Bron, G Venema, K Yamane, J M van Dijl
Bacillus subtilis contains four closely related type I signal peptidases with overlapping substrate specificities. Constitutive and temporally controlled expression of different sip genes.
J Biol Chem: 1997, 272(41);25983-92
[PubMed:9325333]
[WorldCat.org]
[DOI]
(P p)
A Bolhuis, A Sorokin, V Azevedo, S D Ehrlich, P G Braun, A de Jong, G Venema, S Bron, J M van Dijl
Bacillus subtilis can modulate its capacity and specificity for protein secretion through temporally controlled expression of the sipS gene for signal peptidase I.
Mol Microbiol: 1996, 22(4);605-18
[PubMed:8951809]
[WorldCat.org]
[DOI]
(P p)