Difference between revisions of "MurQ"
| Line 30: | Line 30: | ||
<br/><br/> | <br/><br/> | ||
| + | |||
| + | = [[Categories]] containing this gene/protein = | ||
| + | {{SubtiWiki category|[[cell wall degradation/ turnover]]}}, | ||
| + | {{SubtiWiki category|[[utilization of specific carbon sources]]}}, | ||
| + | {{SubtiWiki category|[[phosphoproteins]]}} | ||
| + | |||
| + | = This gene is a member of the following [[regulons]] = | ||
| + | |||
=The gene= | =The gene= | ||
| Line 49: | Line 57: | ||
| − | + | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
Revision as of 13:23, 9 December 2010
- Description: similar to E. coli MurQ (etherase, cleaves lactate from N-acetylmuramic acid)
| Gene name | murQ |
| Synonyms | ybbI |
| Essential | no |
| Product | putative etherase |
| Function | cell wall turnover |
| MW, pI | 32 kDa, 5.404 |
| Gene length, protein length | 912 bp, 304 aa |
| Immediate neighbours | murR, ybbJ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 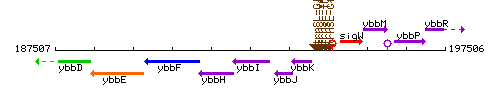
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
cell wall degradation/ turnover, utilization of specific carbon sources, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01700
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: N-acetylmuramic acid 6-phosphate + H2O = N-acetyl-D-glucosamine 6-phosphate + D-lactate (according to Swiss-Prot)
- Protein family: SIS domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on Ser-2 PubMed
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- UniProt: Q45582
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Silke Litzinger, Amanda Duckworth, Katja Nitzsche, Christian Risinger, Valentin Wittmann, Christoph Mayer
Muropeptide rescue in Bacillus subtilis involves sequential hydrolysis by beta-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase.
J Bacteriol: 2010, 192(12);3132-43
[PubMed:20400549]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)