Difference between revisions of "ThrZ"
(→Expression and regulation) |
(→Database entries) |
||
| Line 80: | Line 80: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1TJE 1TJE] (from ''Escherichia coli'', 43% identity, 65% similarity) {{PubMed|15525511}} |
| + | |||
* '''UniProt:''' [http://www.uniprot.org/uniprot/P18256 P18256] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P18256 P18256] | ||
Revision as of 17:16, 18 February 2010
- Description: threonyl-tRNA synthetase (minor)
| Gene name | thrZ |
| Synonyms | thrS2 |
| Essential | no |
| Product | threonyl-tRNA synthetase (minor) |
| Function | translation |
| Metabolic function and regulation of this protein in SubtiPathways: tRNA charging | |
| MW, pI | 73 kDa, 5.753 |
| Gene length, protein length | 1914 bp, 638 aa |
| Immediate neighbours | ywhA, mmr |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 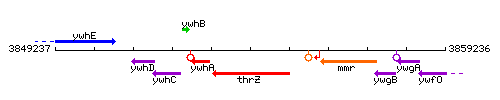
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU37560
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + L-threonine + tRNA(Thr) = AMP + diphosphate + L-threonyl-tRNA(Thr) (according to Swiss-Prot)
- Protein family: class-II aminoacyl-tRNA synthetase family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: P18256
- KEGG entry: [3]
- E.C. number: 6.1.1.3
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Regulatory mechanism:
- T-box: RNA switch, transcriptional antitermination PubMed
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ana Gutiérrez-Preciado, Tina M Henkin, Frank J Grundy, Charles Yanofsky, Enrique Merino
Biochemical features and functional implications of the RNA-based T-box regulatory mechanism.
Microbiol Mol Biol Rev: 2009, 73(1);36-61
[PubMed:19258532]
[WorldCat.org]
[DOI]
(I p)
Helena B Thomaides, Ella J Davison, Lisa Burston, Hazel Johnson, David R Brown, Alison C Hunt, Jeffery Errington, Lloyd Czaplewski
Essential bacterial functions encoded by gene pairs.
J Bacteriol: 2007, 189(2);591-602
[PubMed:17114254]
[WorldCat.org]
[DOI]
(P p)
N Gendron, H Putzer, M Grunberg-Manago
Expression of both Bacillus subtilis threonyl-tRNA synthetase genes is autogenously regulated.
J Bacteriol: 1994, 176(2);486-94
[PubMed:8288542]
[WorldCat.org]
[DOI]
(P p)
H Putzer, N Gendron, M Grunberg-Manago
Co-ordinate expression of the two threonyl-tRNA synthetase genes in Bacillus subtilis: control by transcriptional antitermination involving a conserved regulatory sequence.
EMBO J: 1992, 11(8);3117-27
[PubMed:1379177]
[WorldCat.org]
[DOI]
(P p)