Difference between revisions of "YxeB"
(→Extended information on the protein) |
|||
| Line 75: | Line 75: | ||
* '''Interactions:''' | * '''Interactions:''' | ||
| − | * '''Localization:''' cell membrane (according to Swiss-Prot), extracellular (signal peptide) [http://www.ncbi.nlm.nih.gov/pubmed/18957862 PubMed], membrane [http://www.ncbi.nlm.nih.gov/pubmed/18763711 PubMed] | + | * '''Localization:''' cell membrane (according to Swiss-Prot), extracellular (signal peptide) [http://www.ncbi.nlm.nih.gov/pubmed/18957862 PubMed], membrane, lipid modification as retention signal [http://www.ncbi.nlm.nih.gov/pubmed/18763711 PubMed] |
=== Database entries === | === Database entries === | ||
Revision as of 18:51, 13 February 2010
- Description: hydroxamate siderophore ABC transporter (only ferrioxamine) (binding protein)
| Gene name | yxeB |
| Synonyms | |
| Essential | no |
| Product | hydroxamate siderophore ABC transporter
|
| Function | siderophore uptake |
| MW, pI | 35 kDa, 5.533 |
| Gene length, protein length | 957 bp, 319 aa |
| Immediate neighbours | yxeC, yxeA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 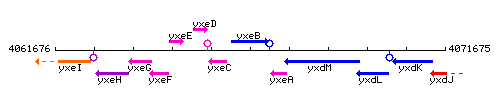
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU39610
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: bacterial solute-binding protein 8 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cell membrane (according to Swiss-Prot), extracellular (signal peptide) PubMed, membrane, lipid modification as retention signal PubMed
Database entries
- Structure:
- UniProt: P54941
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Birgit Voigt, Haike Antelmann, Dirk Albrecht, Armin Ehrenreich, Karl-Heinz Maurer, Stefan Evers, Gerhard Gottschalk, Jan Maarten van Dijl, Thomas Schweder, Michael Hecker
Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis.
J Mol Microbiol Biotechnol: 2009, 16(1-2);53-68
[PubMed:18957862]
[WorldCat.org]
[DOI]
(I p)
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Juliane Ollinger, Kyung-Bok Song, Haike Antelmann, Michael Hecker, John D Helmann
Role of the Fur regulon in iron transport in Bacillus subtilis.
J Bacteriol: 2006, 188(10);3664-73
[PubMed:16672620]
[WorldCat.org]
[DOI]
(P p)
Noel Baichoo, Tao Wang, Rick Ye, John D Helmann
Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon.
Mol Microbiol: 2002, 45(6);1613-29
[PubMed:12354229]
[WorldCat.org]
[DOI]
(P p)
Y Quentin, G Fichant, F Denizot
Inventory, assembly and analysis of Bacillus subtilis ABC transport systems.
J Mol Biol: 1999, 287(3);467-84
[PubMed:10092453]
[WorldCat.org]
[DOI]
(P p)