Difference between revisions of "Rnr"
| Line 54: | Line 54: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU33610&redirect=T BSU33610] | ||
* '''DBTBS entry:''' no entry | * '''DBTBS entry:''' no entry | ||
| Line 91: | Line 92: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU33610&redirect=T BSU33610] | ||
* '''Structure:''' | * '''Structure:''' | ||
Revision as of 14:45, 2 April 2014
- Description: RNase R, required for protection against paraquat stress
| Gene name | rnr |
| Synonyms | yvaJ |
| Essential | no |
| Product | exoribonuclease RNase R (EC 3.1.-.-) |
| Function | nonspecific degradation of rRNA |
| Gene expression levels in SubtiExpress: rnr | |
| MW, pI | 88 kDa, 5.703 |
| Gene length, protein length | 2337 bp, 779 aa |
| Immediate neighbours | smpB, yvaK |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 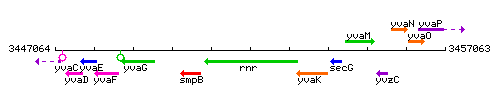
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of nucleotides, Rnases, resistance against oxidative and electrophile stress
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU33610
Phenotypes of a mutant
Database entries
- BsubCyc: BSU33610
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 3'-5'-exoribonuclease
- Protein family: ribonuclease II (RNB) family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU33610
- Structure:
- UniProt: O32231
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
David Bechhofer, Mount Sinai School, New York, USA Homepage
Your additional remarks
References
Andrés Cruz Hernández, Emmanuel Sánchez Millan, Sergio de Jesús Romero Gómez, José Antonio Cervantes Chávez, Rocio Garcia Martínez, Xóchitl Pastrana Martínez, Jackeline Lizzeta Arvizu Gómez, George H Jones, Juan Campos Guillén
Exposure of Bacillus subtilis to mercury induces accumulation of shorter tRNA Cys species.
Metallomics: 2013, 5(4);398-403
[PubMed:23529473]
[WorldCat.org]
[DOI]
(I p)
Alexander Reder, Dirk Höper, Ulf Gerth, Michael Hecker
Contributions of individual σB-dependent general stress genes to oxidative stress resistance of Bacillus subtilis.
J Bacteriol: 2012, 194(14);3601-10
[PubMed:22582280]
[WorldCat.org]
[DOI]
(I p)
Juan Campos-Guillén, Jackeline Lizzeta Arvizu-Gómez, George H Jones, Gabriela Olmedo-Alvarez
Characterization of tRNA(Cys) processing in a conditional Bacillus subtilis CCase mutant reveals the participation of RNase R in its quality control.
Microbiology (Reading): 2010, 156(Pt 7);2102-2111
[PubMed:20360175]
[WorldCat.org]
[DOI]
(I p)
Ji-Hyun Shin, Chester W Price
The SsrA-SmpB ribosome rescue system is important for growth of Bacillus subtilis at low and high temperatures.
J Bacteriol: 2007, 189(10);3729-37
[PubMed:17369301]
[WorldCat.org]
[DOI]
(P p)
Dirk Höper, Uwe Völker, Michael Hecker
Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis.
J Bacteriol: 2005, 187(8);2810-26
[PubMed:15805528]
[WorldCat.org]
[DOI]
(P p)
Irina A Oussenko, Teppei Abe, Hiromi Ujiie, Akira Muto, David H Bechhofer
Participation of 3'-to-5' exoribonucleases in the turnover of Bacillus subtilis mRNA.
J Bacteriol: 2005, 187(8);2758-67
[PubMed:15805522]
[WorldCat.org]
[DOI]
(P p)