Difference between revisions of "BmrA"
| Line 27: | Line 27: | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| − | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=bmrA_3577745_3579514_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:bmrA_expression.png|500px]] | + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=bmrA_3577745_3579514_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:bmrA_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU34820]] |
|- | |- | ||
|} | |} | ||
Revision as of 14:40, 16 May 2013
- Description: multidrug ABC transporter (ATP-binding protein)
| Gene name | bmrA |
| Synonyms | yvcC |
| Essential | no |
| Product | multidrug ABC transporter (ATP-binding protein) |
| Function | multiple antibiotic resistance |
| Gene expression levels in SubtiExpress: bmrA | |
| MW, pI | 64 kDa, 6.474 |
| Gene length, protein length | 1767 bp, 589 aa |
| Immediate neighbours | yvcD, yvzA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 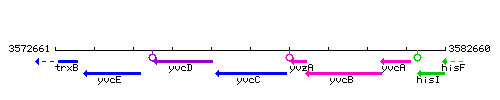
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed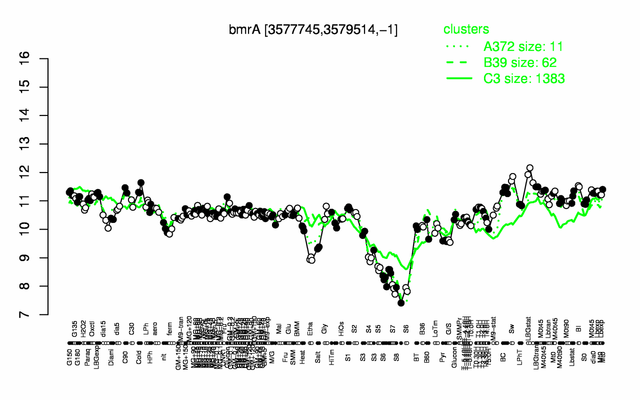
| |
Contents
Categories containing this gene/protein
ABC transporters, resistance against toxins/ antibiotics, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU34820
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: has both a membrane-spanning and an ATP-binding domain PubMed
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: membrane PubMed
Database entries
- Structure:
- UniProt: O06967
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation: constitutively expressed PubMed
- Regulatory mechanism:
- Additional information:
- half-life of the mRNA: 1.5 min PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Kerry J Lee, Lauren M Browning, Tao Huang, Feng Ding, Prakash D Nallathamby, Xiao-Hong Nancy Xu
Probing of multidrug ABC membrane transporters of single living cells using single plasmonic nanoparticle optical probes.
Anal Bioanal Chem: 2010, 397(8);3317-28
[PubMed:20544182]
[WorldCat.org]
[DOI]
(I p)
Cédric Orelle, Francesca Gubellini, Anne Durand, Sergio Marco, Daniel Lévy, Philippe Gros, Attilio Di Pietro, Jean-Michel Jault
Conformational change induced by ATP binding in the multidrug ATP-binding cassette transporter BmrA.
Biochemistry: 2008, 47(8);2404-12
[PubMed:18215075]
[WorldCat.org]
[DOI]
(P p)
Stéphanie Ravaud, Marie-Ange Do Cao, Marie Jidenko, Christine Ebel, Marc Le Maire, Jean-Michel Jault, Attilio Di Pietro, Richard Haser, Nushin Aghajari
The ABC transporter BmrA from Bacillus subtilis is a functional dimer when in a detergent-solubilized state.
Biochem J: 2006, 395(2);345-53
[PubMed:16405427]
[WorldCat.org]
[DOI]
(I p)
Olivier Dalmas, Cédric Orelle, Anne-Emmanuelle Foucher, Christophe Geourjon, Serge Crouzy, Attilio Di Pietro, Jean-Michel Jault
The Q-loop disengages from the first intracellular loop during the catalytic cycle of the multidrug ABC transporter BmrA.
J Biol Chem: 2005, 280(44);36857-64
[PubMed:16107340]
[WorldCat.org]
[DOI]
(P p)
Olivier Dalmas, Marie-Ange Do Cao, Miguel R Lugo, Frances J Sharom, Attilio Di Pietro, Jean-Michel Jault
Time-resolved fluorescence resonance energy transfer shows that the bacterial multidrug ABC half-transporter BmrA functions as a homodimer.
Biochemistry: 2005, 44(11);4312-21
[PubMed:15766260]
[WorldCat.org]
[DOI]
(P p)
Emmanuelle Steinfels, Cédric Orelle, Jean-Raphaël Fantino, Olivier Dalmas, Jean-Louis Rigaud, François Denizot, Attilio Di Pietro, Jean-Michel Jault
Characterization of YvcC (BmrA), a multidrug ABC transporter constitutively expressed in Bacillus subtilis.
Biochemistry: 2004, 43(23);7491-502
[PubMed:15182191]
[WorldCat.org]
[DOI]
(P p)
Cédric Orelle, Olivier Dalmas, Philippe Gros, Attilio Di Pietro, Jean-Michel Jault
The conserved glutamate residue adjacent to the Walker-B motif is the catalytic base for ATP hydrolysis in the ATP-binding cassette transporter BmrA.
J Biol Chem: 2003, 278(47);47002-8
[PubMed:12968023]
[WorldCat.org]
[DOI]
(P p)
Emmanuelle Steinfels, Cédric Orelle, Olivier Dalmas, François Penin, Bruno Miroux, Attilio Di Pietro, Jean-Michel Jault
Highly efficient over-production in E. coli of YvcC, a multidrug-like ATP-binding cassette transporter from Bacillus subtilis.
Biochim Biophys Acta: 2002, 1565(1);1-5
[PubMed:12225846]
[WorldCat.org]
[DOI]
(P p)
Mohamed Chami, Emmanuelle Steinfels, Cédric Orelle, Jean-Michel Jault, Attilio Di Pietro, Jean-Louis Rigaud, Sergio Marco
Three-dimensional structure by cryo-electron microscopy of YvcC, an homodimeric ATP-binding cassette transporter from Bacillus subtilis.
J Mol Biol: 2002, 315(5);1075-85
[PubMed:11827477]
[WorldCat.org]
[DOI]
(P p)
Y Quentin, G Fichant, F Denizot
Inventory, assembly and analysis of Bacillus subtilis ABC transport systems.
J Mol Biol: 1999, 287(3);467-84
[PubMed:10092453]
[WorldCat.org]
[DOI]
(P p)