Difference between revisions of "MtnX"
m (Reverted edits by 134.76.70.252 (talk) to last revision by Jstuelk) |
|||
| Line 100: | Line 100: | ||
* '''Operon:''' ''[[mtnW]]-[[mtnX]]-[[mtnB]]-[[mtnD]]'' [http://www.ncbi.nlm.nih.gov/pubmed/12022921 PubMed] | * '''Operon:''' ''[[mtnW]]-[[mtnX]]-[[mtnB]]-[[mtnD]]'' [http://www.ncbi.nlm.nih.gov/pubmed/12022921 PubMed] | ||
| − | * '''[ | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=mtnX_1428275_1428982_1 mtnX] {{PubMed|22383849}} |
| + | |||
| + | * '''Sigma factor:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/pubmed/12022921 PubMed] | ||
* '''Regulation:''' | * '''Regulation:''' | ||
Revision as of 07:41, 13 April 2012
- Description: 2-hydroxy-3-keto-5-methylthiopentenyl-1-phosphate phosphatase
| Gene name | mtnX |
| Synonyms | ykrX |
| Essential | no |
| Product | 2-hydroxy-3-keto-5-methylthiopentenyl- 1-phosphate phosphatase |
| Function | methionine salvage |
| Metabolic function and regulation of this protein in SubtiPathways: Cys, Met & Sulfate assimilation | |
| MW, pI | 26 kDa, 4.658 |
| Gene length, protein length | 705 bp, 235 aa |
| Immediate neighbours | mtnW, mtnB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 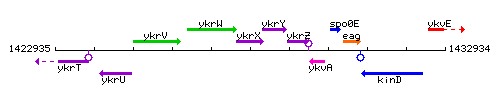
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU13600
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2-hydroxy-5-(methylthio)-3-oxopent-1-enyl phosphate + H2O = 1,2-dihydroxy-5-(methylthio)pent-1-en-3-one + phosphate (according to Swiss-Prot)
- Protein family: MtnX family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 2FEA
- UniProt: O31667
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism: S-box: transcription termination/ antitermination, the S-box riboswitch binds S-adenosylmethionine resulting in termination PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Jerneja Tomsic, Brooke A McDaniel, Frank J Grundy, Tina M Henkin
Natural variability in S-adenosylmethionine (SAM)-dependent riboswitches: S-box elements in bacillus subtilis exhibit differential sensitivity to SAM In vivo and in vitro.
J Bacteriol: 2008, 190(3);823-33
[PubMed:18039762]
[WorldCat.org]
[DOI]
(I p)
Qingping Xu, Kumar Singh Saikatendu, S Sri Krishna, Daniel McMullan, Polat Abdubek, Sanjay Agarwalla, Eileen Ambing, Tamara Astakhova, Herbert L Axelrod, Dennis Carlton, Hsiu-Ju Chiu, Thomas Clayton, Michael DiDonato, Lian Duan, Marc-André Elsliger, Julie Feuerhelm, Slawomir K Grzechnik, Joanna Hale, Eric Hampton, Gye Won Han, Justin Haugen, Lukasz Jaroszewski, Kevin K Jin, Heath E Klock, Mark W Knuth, Eric Koesema, Mitchell D Miller, Andrew T Morse, Edward Nigoghossian, Linda Okach, Silvya Oommachen, Jessica Paulsen, Ron Reyes, Christopher L Rife, Robert Schwarzenbacher, Henry van den Bedem, Aprilfawn White, Guenter Wolf, Keith O Hodgson, John Wooley, Ashley M Deacon, Adam Godzik, Scott A Lesley, Ian A Wilson
Crystal structure of MtnX phosphatase from Bacillus subtilis at 2.0 angstroms resolution provides a structural basis for bipartite phosphomonoester hydrolysis of 2-hydroxy-3-keto-5-methylthiopentenyl-1-phosphate.
Proteins: 2007, 69(2);433-9
[PubMed:17654724]
[WorldCat.org]
[DOI]
(I p)
Agnieszka Sekowska, Valérie Dénervaud, Hiroki Ashida, Karine Michoud, Dieter Haas, Akiho Yokota, Antoine Danchin
Bacterial variations on the methionine salvage pathway.
BMC Microbiol: 2004, 4;9
[PubMed:15102328]
[WorldCat.org]
[DOI]
(I e)
Maumita Mandal, Benjamin Boese, Jeffrey E Barrick, Wade C Winkler, Ronald R Breaker
Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria.
Cell: 2003, 113(5);577-86
[PubMed:12787499]
[WorldCat.org]
[DOI]
(P p)
Ulrike Mäder, Georg Homuth, Christian Scharf, Knut Büttner, Rüdiger Bode, Michael Hecker
Transcriptome and proteome analysis of Bacillus subtilis gene expression modulated by amino acid availability.
J Bacteriol: 2002, 184(15);4288-95
[PubMed:12107147]
[WorldCat.org]
[DOI]
(P p)
Agnieszka Sekowska, Antoine Danchin
The methionine salvage pathway in Bacillus subtilis.
BMC Microbiol: 2002, 2;8
[PubMed:12022921]
[WorldCat.org]
[DOI]
(I e)
Brooke A Murphy, Frank J Grundy, Tina M Henkin
Prediction of gene function in methylthioadenosine recycling from regulatory signals.
J Bacteriol: 2002, 184(8);2314-8
[PubMed:11914366]
[WorldCat.org]
[DOI]
(P p)