Difference between revisions of "HtrB"
| Line 80: | Line 80: | ||
* '''Structure:''' | * '''Structure:''' | ||
| − | * ''' | + | * '''UniProt:''' [http://www.uniprot.org/uniprot/Q9R9I1 Q9R9I1] |
* '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU33000] | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU33000] | ||
Revision as of 14:28, 20 July 2009
- Description: serine protease, response to secretion and heat stresses
| Gene name | htrB |
| Synonyms | yvtA, yvtB |
| Essential | no |
| Product | serine protease |
| Function | response to secretion and heat stresses |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 48 kDa, 4.632 |
| Gene length, protein length | 1374 bp, 458 aa |
| Immediate neighbours | mrgA, cssR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 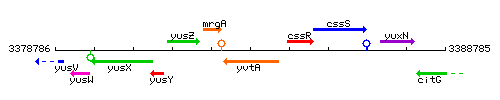
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU33000
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase S1B family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: Q9R9I1
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Hanne-Leena Hyyryläinen, Milla Pietiäinen, Tuula Lundén, Anna Ekman, Marika Gardemeister, Sanna Murtomäki-Repo, Haike Antelmann, Michael Hecker, Leena Valmu, Matti Sarvas, Vesa P Kontinen
The density of negative charge in the cell wall influences two-component signal transduction in Bacillus subtilis.
Microbiology (Reading): 2007, 153(Pt 7);2126-2136
[PubMed:17600057]
[WorldCat.org]
[DOI]
(P p)
Elise Darmon, David Noone, Anne Masson, Sierd Bron, Oscar P Kuipers, Kevin M Devine, Jan Maarten van Dijl
A novel class of heat and secretion stress-responsive genes is controlled by the autoregulated CssRS two-component system of Bacillus subtilis.
J Bacteriol: 2002, 184(20);5661-71
[PubMed:12270824]
[WorldCat.org]
[DOI]
(P p)