Difference between revisions of "QdoI"
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || resistance to plant product quercetin | |style="background:#ABCDEF;" align="center"|'''Function''' || resistance to plant product quercetin | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU39980 qdoI] | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 37,4 kDa, 5.562 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 37,4 kDa, 5.562 | ||
Revision as of 17:42, 7 August 2012
- Description: Fe-containing quercetin 2,3-dioxygenase
| Gene name | qdoI |
| Synonyms | yxaG |
| Essential | no |
| Product | Fe-containing quercetin 2,3-dioxygenase |
| Function | resistance to plant product quercetin |
| Gene expression levels in SubtiExpress: qdoI | |
| MW, pI | 37,4 kDa, 5.562 |
| Gene length, protein length | 1011 bp, 337 aa |
| Immediate neighbours | yxaH, qdoR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 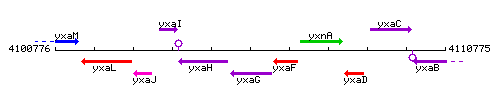
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed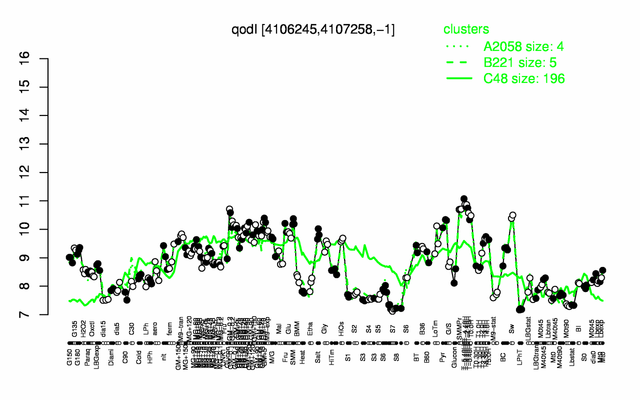
| |
Contents
Categories containing this gene/protein
resistance against other toxic compounds (nitric oxide, phenolic acids, flavonoids, oxalate)
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU39980
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: oxidation of the flavonol quercetin with dioxygen, cleaving the central heterocyclic ring and releasing CO
- Protein family:bicupin family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): Mn(II) (preferred), but also active with Fe(II) snd Co(II) PubMed
- Effectors of protein activity:
Database entries
- Structure: 2H0V
- UniProt: P42106
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
J B Broderick
Catechol dioxygenases.
Essays Biochem: 1999, 34;173-89
[PubMed:10730195]
[WorldCat.org]
[DOI]
(P p)
T D Bugg, J Sanvoisin, E L Spence
Exploring the catalytic mechanism of the extradiol catechol dioxygenases.
Biochem Soc Trans: 1997, 25(1);81-5
[PubMed:9056848]
[WorldCat.org]
[DOI]
(P p)
Original publications
Murugaeson R Kumar, Adrian Zapata, Alejandro J Ramirez, Sara K Bowen, Wilson A Francisco, Patrick J Farmer
Nitrosyl hydride (HNO) replaces dioxygen in nitroxygenase activity of manganese quercetin dioxygenase.
Proc Natl Acad Sci U S A: 2011, 108(47);18926-31
[PubMed:22084064]
[WorldCat.org]
[DOI]
(I p)
Kazutake Hirooka, Yasutaro Fujita
Excess production of Bacillus subtilis quercetin 2,3-dioxygenase affects cell viability in the presence of quercetin.
Biosci Biotechnol Biochem: 2010, 74(5);1030-8
[PubMed:20460727]
[WorldCat.org]
[DOI]
(I p)
Kazutake Hirooka, Satoshi Kunikane, Hiroshi Matsuoka, Ken-Ichi Yoshida, Kanako Kumamoto, Shigeo Tojo, Yasutaro Fujita
Dual regulation of the Bacillus subtilis regulon comprising the lmrAB and yxaGH operons and yxaF gene by two transcriptional repressors, LmrA and YxaF, in response to flavonoids.
J Bacteriol: 2007, 189(14);5170-82
[PubMed:17483215]
[WorldCat.org]
[DOI]
(P p)
Matthew R Schaab, Brett M Barney, Wilson A Francisco
Kinetic and spectroscopic studies on the quercetin 2,3-dioxygenase from Bacillus subtilis.
Biochemistry: 2006, 45(3);1009-16
[PubMed:16411777]
[WorldCat.org]
[DOI]
(P p)
Ken-Ichi Yoshida, Yo-Hei Ohki, Makiko Murata, Masaki Kinehara, Hiroshi Matsuoka, Takenori Satomura, Reiko Ohki, Miyuki Kumano, Kunio Yamane, Yasutaro Fujita
Bacillus subtilis LmrA is a repressor of the lmrAB and yxaGH operons: identification of its binding site and functional analysis of lmrB and yxaGH.
J Bacteriol: 2004, 186(17);5640-8
[PubMed:15317768]
[WorldCat.org]
[DOI]
(P p)
Brett M Barney, Matthew R Schaab, Russell LoBrutto, Wilson A Francisco
Evidence for a new metal in a known active site: purification and characterization of an iron-containing quercetin 2,3-dioxygenase from Bacillus subtilis.
Protein Expr Purif: 2004, 35(1);131-41
[PubMed:15039076]
[WorldCat.org]
[DOI]
(P p)
Laura Bowater, Shirley A Fairhurst, Victoria J Just, Stephen Bornemann
Bacillus subtilis YxaG is a novel Fe-containing quercetin 2,3-dioxygenase.
FEBS Lett: 2004, 557(1-3);45-8
[PubMed:14741339]
[WorldCat.org]
[DOI]
(P p)
Jim M Dunwell, Alan Purvis, Sawsan Khuri
Cupins: the most functionally diverse protein superfamily?
Phytochemistry: 2004, 65(1);7-17
[PubMed:14697267]
[WorldCat.org]
[DOI]
(P p)
Ken-Ichi Yoshida, Izumi Ishio, Eishi Nagakawa, Yoshiyuki Yamamoto, Mami Yamamoto, Yasutaro Fujita
Systematic study of gene expression and transcription organization in the gntZ-ywaA region of the Bacillus subtilis genome.
Microbiology (Reading): 2000, 146 ( Pt 3);573-579
[PubMed:10746760]
[WorldCat.org]
[DOI]
(P p)