Difference between revisions of "FabHB"
| Line 1: | Line 1: | ||
| − | + | ||
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || fatty acid biosynthesis | |style="background:#ABCDEF;" align="center"|'''Function''' || fatty acid biosynthesis | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU10170 fabHB] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/fatty_acid_synthesis.html Lipid synthesis]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/fatty_acid_synthesis.html Lipid synthesis]''' | ||
Revision as of 16:27, 6 August 2012
| Gene name | fabHB |
| Synonyms | yhfB , fabH2 |
| Essential | no |
| Product | beta-ketoacyl-acyl carrier protein synthase III |
| Function | fatty acid biosynthesis |
| Gene expression levels in SubtiExpress: fabHB | |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis | |
| MW, pI | 35 kDa, 5.684 |
| Gene length, protein length | 975 bp, 325 aa |
| Immediate neighbours | yhgE, yhfC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 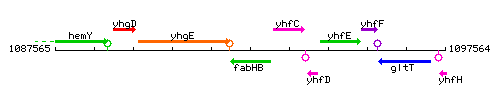
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU10170
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Acetyl-CoA + malonyl-[acyl-carrier-protein] = acetoacyl-[acyl-carrier-protein] + CoA + CO2 (according to Swiss-Prot)
- Protein family: fabH family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: O07600
- KEGG entry: [3]
Additional information
- affinity for butyryl-CoA, but prefers acetyl-CoA in fatty acid biosynthesis PubMed
Expression and regulation
- Operon: fabHB PubMed
- Sigma factor:
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Yasutaro Fujita, Hiroshi Matsuoka, Kazutake Hirooka
Regulation of fatty acid metabolism in bacteria.
Mol Microbiol: 2007, 66(4);829-39
[PubMed:17919287]
[WorldCat.org]
[DOI]
(P p)
Stephen W White, Jie Zheng, Yong-Mei Zhang, Rock
The structural biology of type II fatty acid biosynthesis.
Annu Rev Biochem: 2005, 74;791-831
[PubMed:15952903]
[WorldCat.org]
[DOI]
(P p)
Original Publications
Michaela Wenzel, Malay Patra, Dirk Albrecht, David Y-K Chen, K C Nicolaou, Nils Metzler-Nolte, Julia E Bandow
Proteomic signature of fatty acid biosynthesis inhibition available for in vivo mechanism-of-action studies.
Antimicrob Agents Chemother: 2011, 55(6);2590-6
[PubMed:21383089]
[WorldCat.org]
[DOI]
(I p)
Natalia Martin, Esteban Lombardía, Silvia G Altabe, Diego de Mendoza, María C Mansilla
A lipA (yutB) mutant, encoding lipoic acid synthase, provides insight into the interplay between branched-chain and unsaturated fatty acid biosynthesis in Bacillus subtilis.
J Bacteriol: 2009, 191(24);7447-55
[PubMed:19820084]
[WorldCat.org]
[DOI]
(I p)
Helena B Thomaides, Ella J Davison, Lisa Burston, Hazel Johnson, David R Brown, Alison C Hunt, Jeffery Errington, Lloyd Czaplewski
Essential bacterial functions encoded by gene pairs.
J Bacteriol: 2007, 189(2);591-602
[PubMed:17114254]
[WorldCat.org]
[DOI]
(P p)
Gustavo E Schujman, Luciana Paoletti, Alan D Grossman, Diego de Mendoza
FapR, a bacterial transcription factor involved in global regulation of membrane lipid biosynthesis.
Dev Cell: 2003, 4(5);663-72
[PubMed:12737802]
[WorldCat.org]
[DOI]
(P p)
C Davies, R J Heath, S W White, C O Rock
The 1.8 A crystal structure and active-site architecture of beta-ketoacyl-acyl carrier protein synthase III (FabH) from escherichia coli.
Structure: 2000, 8(2);185-95
[PubMed:10673437]
[WorldCat.org]
[DOI]
(P p)
K H Choi, R J Heath, C O Rock
beta-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis.
J Bacteriol: 2000, 182(2);365-70
[PubMed:10629181]
[WorldCat.org]
[DOI]
(P p)